Answer: Option (A) is the correct answer.
Step-by-step explanation:
A reaction in which nucleus of an atom and a subatomic particle like proton or neutron collide or when any two nuclei combine together then it results in the formation of a new nucleus that is different from its parent nuclei.
For example,
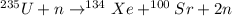
Here, nucleus of Uranium collides with a neutron resulting in formation of a Xenon and Strontium nucleus along with two neutrons.
Therefore, we can conclude that nuclei of an atom participates in the given reaction.