Answer:

Step-by-step explanation:
Here, we want to get an empirical formula
We start by dividing each of the masses by the corresponding atomic masses
The atomic mass of carbon is 12 amu
The atomic mass of hydrogen is 1 amu
We start the division as follows:
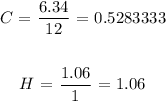
Now, we divide the results by the smaller of the two:

Thus, we have the empirical formula as:
