Answer: The correct option is B.
Step-by-step explanation: We are given that Element X is present in Group 2, so its valence electronic configuration will be:
![[\text{Noble gas}]ns^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/3o2n3xp8hjjllwrm7rsbn2twg8spp3ql6y.png) . It will loose 2 electrons in order to attain stable electronic configuration.
. It will loose 2 electrons in order to attain stable electronic configuration.
Element Y is present in Group 17, so its valence electronic configuration is:
![[\text{Noble gas}]ns^2np^5](https://img.qammunity.org/2018/formulas/chemistry/high-school/3ordowubrxy99ngaa5dt1lq1l5v11twwxx.png) .It will gain 1 electron to attain stable electronic configuration.
.It will gain 1 electron to attain stable electronic configuration.
Hence, we can say that 2 atoms of Y-element will be required to combine with 1 atom of X-element.
Therefore, the formula becomes
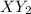 .
.