Answer:
This means that the electric force is approximately 10⁴⁰ times greater than the gravitational force.
Step-by-step explanation:
In your case, you are asked to find the force of attraction due to the mass of the particles and that due to the electric charge.
a) Let us first calculate the force of attraction due to mass, using the law of universal gravitation
Fg = G m1 m2 / r²
With G the universal gravitation constant 6.672 10-11 N m2 / Kg2
For this calculation we have all the data, so we substitute in the equation
Fg = 6,672 10⁻¹¹ 1.7 10⁻²⁷ 9.0 10⁻³¹ / (5 10⁻¹¹)²
Fg = 4.08 10⁻⁻⁴⁷ N
b) Now we calculate the force due to the charges, using Coulomb's law
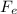 = k q1 q2 / r2
= k q1 q2 / r2
Where k is the Coulomb constant that is worth 8,987 109 N m2 / C2, q is the charge of each particle and r is the distance that separates it, this is the atomic radius
Fe = 8,987 10⁹ (1.6 10⁻¹⁹) (-1.6 10⁻¹⁹) / (5.0 10⁻¹¹)²
Fe = -9.20 10⁻⁷ N
The negative sign indicates that the force is attractive
c) To analyze the difference between the two forces, we divide their values
Fe / Fg = 9.2 10⁻⁷ / 4.08 10⁻⁴⁷
Fe / Fg = 2 10⁴⁰
This means that the electric force is approximately 10⁴⁰ times greater than the gravitational force.