Data:
Molar Mass of CH3OH = 32.04 g/mol
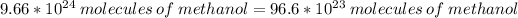
Solving: According to the Law Avogradro, we have in 1 mole of a substance, 6.02x10²³ atoms/mol or molecules
1 mol -------------------- 6.02*10²³ molecules
y mol -------------------- 96.6*10²³ molecules
6.02*10²³y = 96.6*10²³
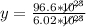
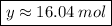
Solving: Find the mass value now
32.04 g ----------------- 1 mol of CH3OH
x g ------------- 16.04 mol of CH3OH
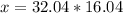

Answer:
The mass is 513.92 grams