Answer: D) an ion that is present in an equilibrium system and a compound added to the system
Explanation: Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in a equilibrium reaction, the equilibrium will shift in a direction so as to minimize the effect.
Thus when a common ion is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing.
For example: In a equilibrium reaction for dissociation of acetic acid:
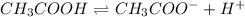
If HCl is introduced wth
 as common ion.
as common ion.
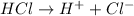
The equilibrium will shift in backward direction where the concentration of
 is decreasing.
is decreasing.