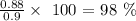Answer:
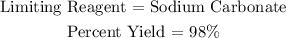
Step-by-step explanation:
The chemical reaction talks about the synthesis of calcium carbonate
It is from the reaction between sodium carbonate and calcium chloride
Let us write the equation of reaction as follows:
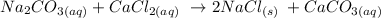
Firstly, we want to get the expected mass of calcium carbonate
This speaks about getting the theoretical yield based on the equation of reaction
From the data collected, 90 ml of 0.20 M (mol/L) of sodium carbonate gave calcium carbonate
We need to get the actual number of moles of sodium carbonate that reacted
We can get this by multiplying the volume by the molarity (kindly note that we have to convert the volume to Liters by dividing by 1000)
Thus, we have it as:
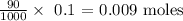
Hence, we see that 0.009 moles of sodium carbonate reacted theoretically
Since 1 mole of sodium carbonate gave 1 mole calcium carbonate, it is expected that 0.009 mole of sodium carbonate will give 0.009mole of calcium carbonate
What we have to do now is to get the theoretical grams of calcium carbonate produced
That would be the product of the number of moles of calcium carbonate and its molar mass
The molar mass of calcium carbonate is 100 g/mol
The theoretical yield (expected mass) is thus:
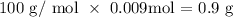
Finally, we proceed to get the percentage yield which is calculated using the formula below:
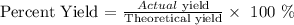
The actual yield is the observed mass which is given as 0.88 g
The percent yield is thus: