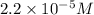Answer : The hydroxide ion concentration in a solution of ammonia is,
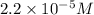
Explanation : Given,
pOH = 4.65
pOH : It is defined as the negative logarithm of hydroxide ion concentration.
Mathematically it is represented as :
![pOH=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tyqnxkdounrtow0llesefga8x48nlscnj6.png)
![4.65=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/8n7czcxcygoim28ohmusrlodjolrj7jszh.png)
![[OH^-]=2.2* 10^(-5)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/rwkywmbhszu7d4hltfz8k3zivwjydc5urt.png)
Therefore, the hydroxide ion concentration in a solution of ammonia is,