Given:
Atm = 1.00 atm
Temperature = 0.00°C
Amount of gas = 1 mole which occupues 22.4 L
Let;s find the number of molecules of an ideal gas are in one cm^3 under these conditions.
We have:
1 mole of ideal gas = 22.4 L
This is called the molar volume of gas.
To find the amount of molecules, apply the avogrado's constant:
Number of molecules in 1 mol = 6.023 x 10²³
Hence, for 1 cm³, we have:
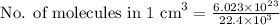
Solving further:
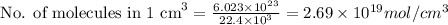
Therefore, the amount of molecules of an ideal gas in one cm^3 under these conditions is:
2.69 x 10¹⁹ mol/cm^3
ANSWER:
C. 2.69 x 10¹⁹