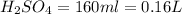Answer: Part A:
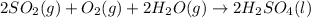
Part B: Volume of
 =0.16L
=0.16L
Explanation:
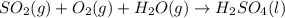
According to law of conservation of mass, the atoms on product side must be equal to the atoms on reactant side so that the mass remains conserved.
Part A: The balanced chemical reaction is:
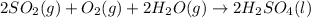
Part B: 1 mole of
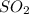 occupies 22.4 L at STP
occupies 22.4 L at STP
2 moles of
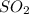 will occupy
will occupy
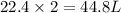 and produce 2 moles of
and produce 2 moles of

mass of 2 moles of
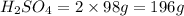
Thus 44.8 L of
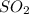 will produce 196 g of
will produce 196 g of

67.2 L of
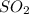 will produce
will produce
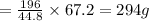 of
of

mass of
 = 294 g
= 294 g
density of
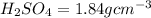
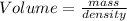
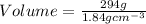
Thus Volume of