Answer: The bond angle in methane compound is 109.5°
Step-by-step explanation:
The chemical formula of methane molecule is
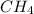
The equation used to calculate hybridization follows:
![\text{Number of electron pair}=(1)/(2)[V+N-C+A]](https://img.qammunity.org/2018/formulas/chemistry/college/zia3jm1s1xbt3kxm0r43raz35v736wepqh.png)
where,
V = number of valence electrons present in central atom
, carbon = 4
N = number of monovalent atoms bonded to central atom = 4
C = charge of cation
A = charge of anion
Putting values in above equation, we get:
![\text{Number of electron pair}=([4+4])/(2)=4](https://img.qammunity.org/2018/formulas/chemistry/high-school/wpptzqnqjflyohqz8j107c0ssida2w7hix.png)
The number of electron pair are coming out to be 4. This means that the hybridization will be
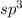 and the electronic geometry of the molecule will be tetrahederal.
and the electronic geometry of the molecule will be tetrahederal.
The bond angle between the atoms when the electronic geometry is tetrahederal is 109.5°
The image of the methane molecule is attached below.
Hence, the bond angle in methane compound is 109.5°