The mass of 0.0750 moles of calcium is 3.01g.
To calculate the mass of calcium we need to use the molar mass that we can find in the Periodic Table of Elements.
The molar mass of calcium is 40.1 g/mol.
Now, with a mathematical Rule of Three we can calculate the mass of calcium of 0.0750 moles:
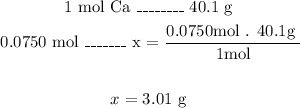
So, the mass of 0.0750 moles of calcium is 3.01g.