Answer : The correct option is, 4) the identity of the substance does not change.
Explanation :
Chemical change : It is a change where changes occurs in the chemical composition and thus resulting in the formation of new compound or substance. It is usually an irreversible reaction.
Physical change : It is a change in which shape and size of a substance changes. In this there is no change in the chemical composition of the substance and no new substance is forming. It is usually an reversible reaction.
As we know that evaporation is a process in which the phases changes from liquid state to gaseous state at constant temperature.
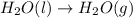
As per question, when water evaporates, the liquid water changes to gaseous form then the identity of the substance does not change. This is a physical change because only phase changes.
Hence, the evaporating water is an example of a physical change because the identity of the substance does not change.