Answer: The correct answer is option (B).
Step-by-step explanation:
Law of conservation of mass: 'In a chemical reaction, mass neither be created nor be destroyed'
In the balance chemical equation,total mass of the reactants is equal to the total mass of the products.
A.
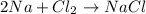
![[2* (23 g/mol)+(35.5 g/mol)* 2]=[23 g/mol+35.5 g/mol]](https://img.qammunity.org/2018/formulas/physics/high-school/74s0y8pdpsa7p2wxqszby4ltakqy0ik7po.png)
117 g/mol ≠ 58.5 g/mol
Law of Conservation of Mass not followed.
B.
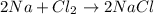
![[2* (23 g/mol)+(35.5 g/mol)* 2]=2* [23 g/mol+35.5 g/mol]](https://img.qammunity.org/2018/formulas/physics/high-school/1q6s2o04sfdjv5h2wd64ppcs2wc1lcwc9d.png)
117 g/mol = 117 g/mol
Law of Conservation of Mass is followed.
C.
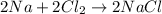
![[2* (23 g/mol)+(35.5 g/mol)* 2]=2* [23 g/mol+35.5 g/mol]](https://img.qammunity.org/2018/formulas/physics/high-school/1q6s2o04sfdjv5h2wd64ppcs2wc1lcwc9d.png)
188 g/mol ≠ 117 g/mol
Law of Conservation of Mass not followed.
D.
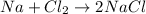
![[(23 g/mol)+(35.5 g/mol)* 2]=2* [23 g/mol+35.5 g/mol]](https://img.qammunity.org/2018/formulas/physics/high-school/t6zjfltnkn41m0r6o0kwbbxkeemewegwcm.png)
94 g/mol ≠ 117 g/mol
Law of Conservation of Mass not followed.
Hence, the correct answer is option (B).