The reaction presented to us is balanced since we have 4 aluminum atoms and 6 oxygen atoms on both sides of the reaction.
Now, we have a given mass of aluminum and oxygen, we must first determine the moles of each using their molar mass.
Moles of Al
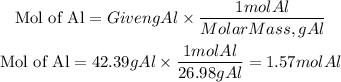
Moles of O2
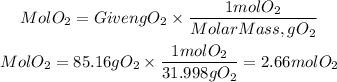
Now that we have the number of moles, we will calculate what the limiting reagent is, that is, the reagent that limits the reaction by its number of moles.
To find the limiting reactant we must compare the amount of product obtained with the given amount of reactant separately. The reactant that produces the least amount of product is the limiting reactant.
Using Al as limiting reactant
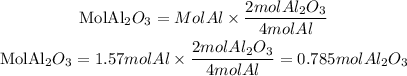
Using O2 as a limiting reactant
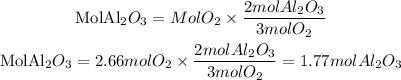
Aluminum is the reagent that produces the least amount of aluminum oxide, so the limiting reagent will be Al. And it will produce 0.785 moles of Al2O3. In grams this will be:
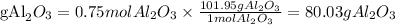
The percent yield will be:
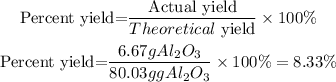
The percent yield will be 8.33%