Answer: The molar mass of the element is 80.8 g/mol
Step-by-step explanation:
We are given:
Number of atoms =
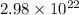
According to mole concept:
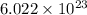 number of atoms are contained in 1 mole of an element
number of atoms are contained in 1 mole of an element
So,
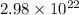 number of atoms will be contained in
number of atoms will be contained in
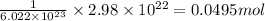 of an element
of an element
To calculate the molar mass for given number of moles, we use the equation:
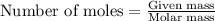
Given mass of element = 4.00 g
Moles of an element = 0.0495 moles
Putting values in above equation, we get:
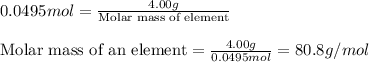
Hence, the molar mass of the element is 80.8 g/mol