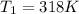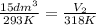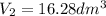Answer:
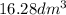
Explanation: Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
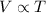 (At constant pressure and number of moles)
(At constant pressure and number of moles)
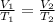
where V= volume
T= temperature in Kelvin
Given:
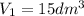
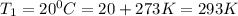
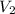 =?
=?