Answer:
D. 2 and 3 only.
Step-by-step explanation:
First let's remember the Le Chatelier's Principle: Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish equilibrium.
Let's see the reaction:
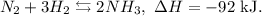
You can note that this reaction is exothermic. If we want the equilibrium to move to the left, we have to increase the temperature. In this case, we don't want that because we want to increase the concentration of NH3 and this means that the equilibrium goes to the right, so we have to decrease the temperature.
In the case of pressure, if we want to reach the equilibrium to the right, where is NH3, we have to increase the pressure because the pressure will move in such a way as to decrease the pressure again.
The answer would be D. 2 and 3 only.