Answer:
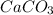
Explanation:-
A compound is a pure substance which is made from atoms of different elements combined together in a fixed ratio by mass.
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Here calcium is having an oxidation state of +2 and carbonate is a polyatomic ion with oxidation state of -2. Thus they combine to form neutral
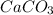 .
.
The chemical formula of an ionic compound is written as
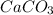 .
.