Answer: C. The molarity of hydronium is lower than the molarity of hydroxide.
Explanation: A basic solution is one which has pH above 7 and an acidic solution is one which has pH below 7.
A basic solution has more number of
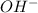 ions as compared to
ions as compared to
 ions and an acidic solution has lesser number of
ions and an acidic solution has lesser number of
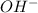 ions as compared to
ions as compared to
 ions .
ions .
A neutral solution has equal number of
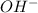 ions as compared to
ions as compared to
 ions.
ions.