Answer:
B. 0.2 M
Step-by-step explanation:
Hello,
In this case, by considering that the molarity is defined as:
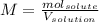
We replace the given 1 mole of HCl as the moles of the solute and 5 liters as the volume of the solution to obtain the required molarity as shown below:
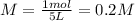
Therefore, the answer is B. 0.2 M.
Best regards.