Answer:
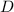
Step-by-step explanation:
Here, we want to get the definition of a weak base
A weak base is a base that does not dissociate completely in solution.
What this means is that when the base is placed in a solution, its dissociation into the corresponding metallic and hydroxide ions is incomplete