Answer: The ionization energy decreases down the group.
Step-by-step explanation:
Ionization energy is defined as the energy required to remove an electron from the outermost shell of an isolated gaseous atom. It is represented as
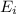
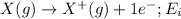
Ionization energy decreases on moving down the group. This happens because the number of shells increases as we move down the group. The electrons get added in the new shell.
This results in the shielding of outermost electrons more from the inner ones, which decreases the attraction between the outermost electrons and the nucleus. Hence, the removal of electron from the outermost shell becomes easy and requires less energy.
Hence, the ionization energy decreases down the group.