Answer:
Lewis structure are defined structure which represents the number of valence electrons present around the atom in a molecule. They are known as Lewis electron dot structures.
Valence electrons are the electrons present in the valence shell of an atom.
A) Lithium has total three electrons out of which one is valence electron as we see that from its electronic configuration.
[Li]=
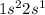 , For Lewis structure see figure (I)
, For Lewis structure see figure (I)
B) Sodium has total 11 electrons out of which one is valence electron as we can see that from its electronic configuration.
[Na]=
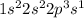 For Lewis structure see figure (II)
For Lewis structure see figure (II)
C) In sodium cation, there are total 10 electrons, since it will easily loose its one valence electron to attain noble gas configuration by which there will be no valence electron present in it. So, its electronic configuration becomes
![[Na^+]=1s^22s^22p^63s^0](https://img.qammunity.org/2018/formulas/chemistry/high-school/8gb0rkaqxyku38nu267cb2sz60l8zg5zfc.png) For Lewis structure see figure (III)
For Lewis structure see figure (III)
D) In fluorine anion, there are total 10 electrons out of which 9 electrons are of its own and one electron is being added in its valence shell ,so that it can attain noble gas configuration.
![[F]=1s^22s^22p^5](https://img.qammunity.org/2018/formulas/chemistry/college/2k2wis2nzyi9vq34qm9xm0n60ypl65bck9.png)
![[F^-]=1s^22s^22p^6](https://img.qammunity.org/2018/formulas/chemistry/college/y66vgwqofshzslf69xxnok1k6s8kouce6z.png) For Lewis structure see figure (IV)
For Lewis structure see figure (IV)