Answer
NO is the limiting reactant
Step-by-step explanation
Given that:
The mass of NH3 that reacted = 43.4 g
The mass of NO that reacted = 30 g
The equation for the reaction is: 4NH3 + 6NO --> 5N2 + 6H2O
What to find:
The limiting reactant.
Step-by-step solution:
The first step is to convert the given mass of the reactants to moles.
Using the mole formula and the molar masses of (NH3 = 17.0 g/mol and NO = 30.0 g/mol)
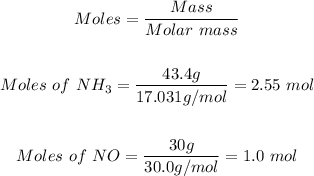
The final step is to determine the limiting reactant by comparing the mole ratio from the given equation with the mole ratio in the step above.
From the equation, 4 moles of NH3 reacted with 6 moles of NO
So 2.55 moles of NH3 is expected to react with (2.55 x 6)/4 = 3.825 moles of NO
1.0 mol NO is less than 3.825 mol NO, hence, NO is the limiting reactant because it will be the reactant to be completely used up first.