Answer:
Polar bond.
Step-by-step explanation:
Hello,
In this case, since water involves two bonds hydrogen to oxygen, the way for us to identify its type is via the electronegativity difference. In such a way, that difference is computed below:
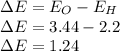
Such value means that the resulting bond is polar because it is greater than 0.7 which is the limit for nonpolar bonds and less than 1.7 which is the limit for ionic bonds.
Best regards.