Answer:
Step-by-step explanation:
As the cell has a copper strip like the anode and a coin or nail (we can consider this like it is nickel) like the cathode, we can write both half-reactions:
• Copper half-reaction,:
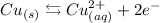
In the copper anode, the oxidation reaction takes place. This is the positive terminal.
• Nickel half-reaction,:
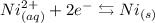
In the nickel cathode, the reduction reaction takes place. This is the negative terminal.
The direction of the electron flow always go from the anode to the cathode.