Answer: The chemical equation is given below.
Step-by-step explanation:
Every balanced chemical equation follows Law of Conservation of mass. This law states that the total number of individual atoms on reactant side must be equal to the total number or individual atoms on the product side.
We need to write the chemical equation for the reaction of sulfur trioxide with water, the equation follows:
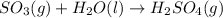
By Stoichiometry of the reaction:
1 mole of sulfur trioxide gas reacts with 1 mole of water molecule to produce 1 mole of sulfuric acid gas.
Hence, the balanced chemical equation is given above.