Answer: Option (C) is the correct answer.
Step-by-step explanation:
A chemical reaction in which more reactive metal or substance displaces one less reactive metal or substance then this type of reaction is known as single displacement reaction.
Thus, we can conclude that out of the given options, the reaction equation
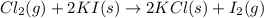
As in this reaction, one chlorine atom replaces one iodine atom hence it is a single displacement reaction.