I'll Be Solving Two Different Questions.
Formula For Both :
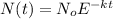
1. A 40 gram sample of a substance thats used for drug research has a k-value of 0.1455
![\left[\begin{array}{ccc}N(t)=40E^(-0.1455t)\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/sdhalvmgoxypnhyzwwufpvnmzlk4xtuhl1.png)
2. A 40 gram sample of a substance thats used for drug research has a k-value of 0.1446.
![\left[\begin{array}{ccc}N(t)=40E^(-0.1446t)\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/lx4e5m22nnqt30naclmox8pdwxhvq0ktls.png)
![\left[\begin{array}{ccc}Number&1\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/4ver9eylvyt35eyf5fcc7xpbm3hz08cuhi.png)
We Determine That 20 Is Half Of 40.
With That Being Said
![\left[\begin{array}{ccc}N(t)=40E^(-0.1455t)&=20\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/8lz8em5o6qmz7v3hiolgv287tk8qilhbo8.png)
![\left[\begin{array}{ccc}In(E^(-0.1455))=(In(1)/(2) )/(-0.1455) \end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/c7qsp9397wags3j7grix9ceqk3f220cwsr.png)
![\left[\begin{array}{ccc}T=(In(1)/(2) )/(-0.1455)&Or&T=(-In(2))/(0.1455)\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/v6u9dfs7779c21kevtp7tvi45po6b011zt.png)
![\left[\begin{array}{ccc}-0.5In/0.1455\\=4.76\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/tmyv7yhlxbzl3ggjqjo9wqanfq133lev5t.png)
Rounding It To Be [ 4.8 ]
![\left[\begin{array}{ccc}Number&2\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/mwyjjp2xupfozzjshrj5eexmfhduof6l4m.png)
Again 20 Is Determined To Be Half Of 40
With That Being Said
![\left[\begin{array}{ccc}N(t)=40E^(-0.1446t)&=20\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/hbe57zkahic1doo8yhl2fayvmrkoi53y2x.png)
![\left[\begin{array}{ccc}In(E^(-0.1446))=(In(1)/(2) )/(-0.1446) \end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/sxn7hndynik0q3k0p6r7ftvy1ptx8ho16n.png)
![\left[\begin{array}{ccc}T=(In(1)/(2) )/(-0.1446)&Or&T=(-In(2))/(0.1446)\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/b5h9iq8ek31nlyau0j6ajo7qtz4mahngj0.png)
![\left[\begin{array}{ccc}-0.5In/0.1446\\=4.79\end{array}\right]](https://img.qammunity.org/2018/formulas/mathematics/high-school/svq7imvzux69kpwlxe0ugw9mcjmb5zdz8l.png)
Rounding It To Be [ 4.8 ]