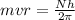Answer:
As we know that as per Rutherford's model we have will have an model in which he said that there is a small center at which whole positive charge is concentrated in an atom and all electrons will revolve around that positive charge.
So here in Rutherford's model he describe about the nucleus of the atom.
Now in Bohr's model he said that the orbit in which all electrons revolve around the nucleus is known as stationary orbit and there is no energy loss in that orbit when electron revolves in it.
So here we can say that in Bohr's model he included the part of stationary orbit which is not included in Rutherford's model.
As we can say that in Bohr's model the energy of electron is constant when it revolves in its stationary orbit and when electron changes its orbit then energy will be released in form of photons.
For stationary orbit the angular momentum of the electron must be integral multiple of
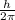
so it is