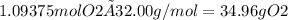Final answer:
To react with 10.0 grams of octane in a combustion reaction, 34.96 grams of oxygen are required. This is determined using the balanced chemical equation and molar masses of octane and oxygen.
Step-by-step explanation:
The question concerns the stoichiometry of the reaction between octane (C8H18) and oxygen (O2) during combustion. To determine the grams of oxygen required, we will use the balanced chemical equation and the molar mass of the reactants. The balanced equation for the combustion of octane is:
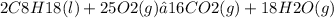
From the equation, we understand that for every 2 moles of octane, 25 moles of oxygen are needed. The molar mass of octane is 114.23 g/mol and the molar mass of oxygen is 32.00 g/mol. Calculating the moles of octane for 10.0 grams gives us:
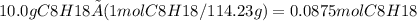
Using the stoichiometry of the reaction, the moles of oxygen required is:
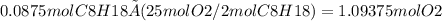
Finally, converting moles of oxygen to grams: