Answer : It is called as standard heat of formation.
Explanation :
Enthalpy of formation : The enthalpy of change when one mole of a pure compound is formed from its elements is called the enthalpy of formation. It is denoted as,
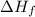 .
.
Standard heat of formation : When the reacting elements and the product formed are all in their standard state at
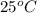 , the enthalpy change accompanying the chemical reaction is called the standard enthalpy of formation or standard heat of formation. It is denoted as,
, the enthalpy change accompanying the chemical reaction is called the standard enthalpy of formation or standard heat of formation. It is denoted as,
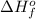 .
.
Hence, it is called as standard heat of formation.