Answer:
I don't know why 27.6 g is not the correct answer. I believe that it is correct.
27.6 grams of
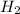 .
.
Step-by-step explanation:
The balanced equation tells us that 17 moles of hydrogen will react completely with 8 moles of carbon monoxide. That is a molar ratio of 17/8 moles H2/molesCO.
If we start with 6.5 moles of CO, we need:
(6.5 moles CO)*(17/8 moles H2/moles CO) = 13.8 moles of H2 (3 sig figs)
Convert 13.8 moles of H2 into grams H2 by multiplying by the molar mass iof H2 (2g/mole):
(13.8 moles H2)*(2 g H2/mole H2) = 27.6 grams of
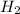 .
.