Answer:
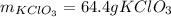
Step-by-step explanation:
Hello,
At first, the undergoing chemical reaction is:
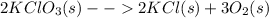
As 15.0 L of oxygen were produced, one computes this amount in moles considering STP conditions (0°C and 1 atm) for the ideal gas equation as shown below:
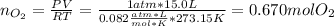
Now, since those moles are the actual obtained moles, one computes the theoretical moles of oxygen which are more precisely associated with the needed grams of potassium chlorate as follows:
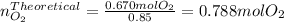
Finally, we apply the stoichiometry to determine the moles of potassium chlorate as shown below:
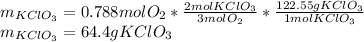
Best regards.