Answer: The correct answer is Option C.
Step-by-step explanation:
A beta particle is released when a nucleus undergoes a beta decay process. In this process, a neutron gets converted to a proton and an electron. The electron released is known as beta particle only.
The equation that represents beta decay process is:
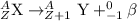
The charge that this particle carries is -1 and it has a mass of 0 units. It is represented as
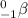 of
of
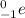
Hence, the correct answer is Option C.