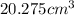Answer: The molar volume of carbon dioxide is
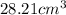 and that of ammonia is
and that of ammonia is
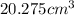
Step-by-step explanation:
To calculate the molar volume for the given densities, we use the mass to be molar mass.
To calculate volume of a substance, we use the equation:
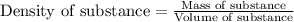 ......(1)
......(1)
Density of carbon dioxide =
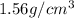
Molar mass of carbon dioxide = 44.01 g
Putting values in equation 1, we get:
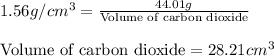
Hence, the molar volume of carbon dioxide is
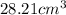
Density of ammonia =
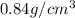
Molar mass of ammonia = 17.031 g
Putting values in equation 1, we get:
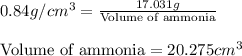
Hence, the molar volume of ammonia is