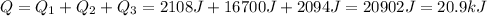We should divide the problem into 3 separate processes.
1) Bring the temperature of the ice from
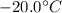
to its melting point (
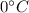
): the amount of heat needed in this process is
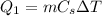
where
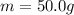
is the mass of the ice
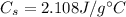
is the specific heat capacity of ice
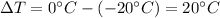
is the increase of temperature
Plugging numbers into the equation, we find
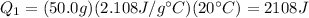
2) Fusion of ice
When the ice is at melting point, we need to add a certain amount of heat in order to melt it, and this amount of it is given by:
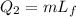
where
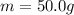
is the mass of ice
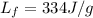
is the latent heat of fusion of ice
Plugging numbers into the equation, we find
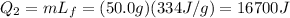
During this phase transition, the temperature of the ice/water does not change.
3) Bring the temperature of the water from
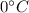
to
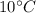
The amount of heat needed for this process is
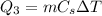
where
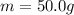
is the mass of water
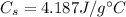
is the specific heat capacity of water
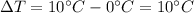
is the increase of temperature
Plugging numbers into the equation, we find
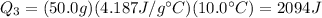
--> therefore, the total energy needed for the whole process is: