Answer : The volume of helium gas is, 244.72 liters
Explanation : Given,
Mass of helium gas = 43.7 g
Molar mass of helium gas = 4 g/mole
As we know that at STP, 1 mole of gas contains 22.4 liter volume of gas.
First we have to calculate the moles of helium gas.
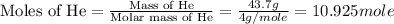
Now we have to calculate the volume of helium gas.
As, 1 mole of helium gas contains 22.4 liter volume of gas
So, 10.925 mole of helium gas contains
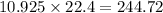 liter volume of gas
liter volume of gas
Therefore, the volume of helium gas is, 244.72 liters