Answer:
option A
Explanation:
given,
Volume of gas(V₁) = 16 cm³
Pressure of the gas(P₁) = 20 L/cm²
Volume of Gas(V₂) when pressure(P₂) is 2.2 L/cm²
considering temperature constant
P₁ V₁ = P₂ V₂
20 × 16 =2.2 × V₂
V₂ =
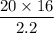
V₂ = 145.45 cm³
hence, the correct answer is option A