Answer:
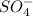
Step-by-step explanation:
The sodium sulfate compound corresponds to a water soluble salt.
When a salt dissolves it will dissociate into the ions that compose it.
It dissociates into Cations (positively charged ions) and anions (negatively charged ions)
Sodium sulfate formula
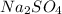
Sodium sulfate when dissolved in water
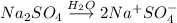
Ions present in the solution:
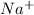 Cation
Cation
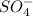 Anion
Anion