Answer:
0.893 Faraday
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is given as follows:

Now, the oxygen's half-reaction turn out into:
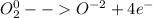
Let us identify that four electrons were involved since there are two oxygens that transfer two electrons for a total of four. In such a way, to compute the faradays of electricity, one multiplies the changing moles by the amount of transferred electrons as shown below:
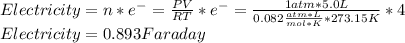
Best regards.