Answer:
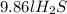
Step-by-step explanation:
STP means standard conditions of temperature and pressure and under these conditions, one mole of any substance occupies a volume of 22.4 l.
We have
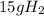 to calculate the number of mols you must calculate the molecular mass of the compound.
to calculate the number of mols you must calculate the molecular mass of the compound.
H 2 (1g / mol)
S 32 g / mol
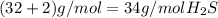
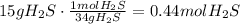
Now we calculate the volume occupied by
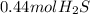
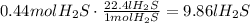
the volume of 15.0 g of
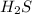 is 9.86l.
is 9.86l.