Answer: Option (A) is the correct answer.
Step-by-step explanation:
A catalyst is a substance that participates in a chemical reaction without itself getting consumed and helps in increasing the rate of a reaction.
For example,
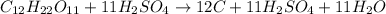
Here, in this reaction sulfuric acid does not get consumed and remains the same on both reactant and product side.
Hence, it acts as a catalyst. Whereas carbon only exists as a solid substance in its elemental state. Therefore, C on product side is a solid.
On the other hand, water can exist as solid, liquid or gas.
Hence, we can conclude that water exists as gaseous product in this reaction.