Answer: The unknown noble gas is Krypton.
Step-by-step explanation:
Rate of effusion is defined as the amount of volume displaced per unit time.
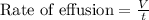
To calculate the rate of diffusion of gas, we use Graham's Law.
This law states that the rate of effusion or diffusion of gas is inversely proportional to the square root of the molar mass of the gas. The equation given by this law follows the equation:
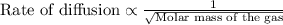
We are given:
Time taken by neon gas = 72 s
Time taken by unknown gas = 147 s
Molar mass of neon gas = 20.18 g/mol
By taking their ratio, we get:
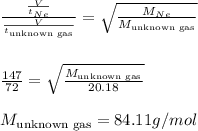
The noble gas having molar mass of 84.11 g/mol is Krypton
Hence, the unknown noble gas is Krypton.