Answer: The hydrogen gas is the limiting reagent and 6 molecules of water are produced.
Step-by-step explanation:
We are given:
Molecules of hydrogen gas = 6 molecules
Molecules of oxygen gas = 6 molecules
The chemical equation for the reaction of hydrogen gas and oxygen gas follows:
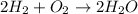
By Stoichiometry of the reaction:
2 molecules of hydrogen gas reacts with 1 molecule of oxygen gas
So, 6 molecules of hydrogen gas will react with =
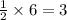 molecules of oxygen gas
molecules of oxygen gas
As, given amount of oxygen gas is more than the required amount. So, it is considered as an excess reagent.
Thus, hydrogen gas is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
2 molecules of hydrogen gas produces 2 molecules of water
So, 6 molecules of hydrogen gas will produce =
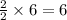 molecules of water
molecules of water
Hence, the hydrogen gas is the limiting reagent and 6 molecules of water are produced.