Answer : The mass percent of fluorine in the compound is, 47.36%
Explanation :
Mass percent : It is defined as the mass of the given component present in the total mass of the compound.
Formula used :
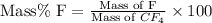
First we have to calculate the mass of fluorine.
Mass of fluorine = Mass of
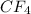 - Mass of C
- Mass of C
Mass of fluorine = 171 - 90 = 81 g
Now put all the given values in this formula, we get the mass percent of fluorine in the compound.
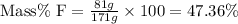
Therefore, the mass percent of fluorine in the compound is, 47.36%