Answer:
Both of them have 1 mole of zinc.
Step-by-step explanation:
Hello,
In this case, if we analyze these two situations via stoichiometry, we are going to realize there is 1 mol of zinc in both of them as shown below:
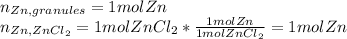
This is substantiated by knowing there is only one zinc atom in the zinc chloride, therefore, one mole is found as well as in the zinc granules which is just pure zinc.
Best regards.