Answer:
C.
Step-by-step explanation:
We have to keep in mind for this question that we have to take into account the subscripts.
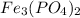
The subscript will affect only the number in the left, so the first part would be:
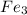
In this part we will have 3 iron atoms. Then we will have:
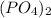
In the
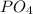 we will have 1 atom of P and 4 atoms of O. (When we dont have any number as subscript we have to assume that we have a "1")
we will have 1 atom of P and 4 atoms of O. (When we dont have any number as subscript we have to assume that we have a "1")
Then we have a "2" out side the the parenthesis
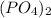 , so we have to multiply by "2" all the atoms inside the parenthesis, so:
, so we have to multiply by "2" all the atoms inside the parenthesis, so:
1 atom of P x 2 = 2
4 atoms of O x 2 = 8
So, in total we will have:
3 atoms of Fe + 8 atoms of O + 2 atoms of P = 13 atoms