Let us first define what density is. Density is defined as the mass of a substance that occupies a certain volume. It is described by the following equation:
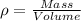
Now, we have the volume of the liquid that will be equal to the volume of the container, that is, 160.0 cm3.
The mass of the liquid will be equal to the total mass (Container + Liquid) minus the mass of the container. The mass of the liquid is:
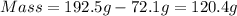
Now, we substitute the value of mass and volume and find the density:
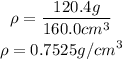
Answer: The density of the liquid is 0.7525 g/cm^3